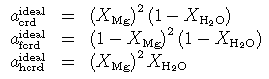
With the site distributions for Fe--Mg cordierites as follows,
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
the ideal mixing activities are given as
and coded up for THERMOCALC,
% =============== Ideal mixing in hydrous cordierites =============
cd 3
crd 1 2 1 1 -1 1 2 1 1 -1 2 1
fcrd 1 2 0 1 1 1 2 1 1 -1 2 1
bulk 1 1 0 1 1 1
hcrd 1 2 1 1 -1 1 2 0 1 1 2 1
bulk 1 1 0 1 1 2
x(cd) 0.3
h(cd) 0.1
The thermodynamic properties of the hydrous cordierite end-member have been updated slightly from HP90 to take account of the measured heat capacities of Carey (1993) and the enthalpy of hydration measured by Carey & Navrotsky (1992), as well as the new water content data of Skippen & Gunter (1996). Taking the heat capacity of hydrous cordierite as 8.3 J larger than anhydrous cordierite (Carey, 1993), together with the assumption of 1 mole of H2O in the hydrous end-member, leads to
for the equilibrium hcrd = crd + H2O. A least squares fit to the hydration data of Mirwald et al. (1979) and Skippen & Gunter (1996) yields an enthalpy of reaction which is within error of the value of 41.8 ±1.6 kJ measured calorimetrically by Carey & Navrotsky (1992). It was therefore decided to accept the calorimetric enthalpy as a constraint and to retrieve the entropy from the hydration data. The resulting values used in the data set are
for the reaction crd + H2O = hcrd. The level of agreement between the calorimetric data and the measured water contents makes a satisfactory justification of the assumption of ideal mixing of cordierite and hydrous cordierite with one mole of H2O, and lends weight to the model as a tool for calculating the thermodynamic properties of water-bearing cordierites.
|
|