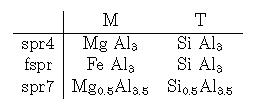
Sapphirines in the MAS system lie close to a solid solution Mg{4-y}Al{4+y}) [Al{4+y} Si{2-y}] with common sapphirines lying between the 442 (y = 0) and 793 (y = 0.5) compositions (e.g. Higgins & Ribbe, 1979). These two compositions are used as end-members in this study, here called spr4 (Mg4Al8Si2O20) and spr7 (Mg3.5Al9Si1.5O20). According to Moore (1969), Higgins & Ribbe (1979) and Christy et al. (1992) there are high degrees of long and short range order on the 8 octahedral and 6 tetrahedral sites, although the details are incompletely known. The consensus of these studies is that Mg resides on the M4, M5 and M6 sites with Al filling M7, leaving the remaining four octahedral sites (M1, M2, M3, M8) on which Mg and Al can both substitute. Of the 6 tetrahedral sites, T2 is occupied by Si while T5 is occupied by Al, leaving the four remaining tetrahedral sites (T1, T3, T4, T6) on which Al and Si can substitute. There is some suggestion that M1, M2 and M8 may be preferred by Al, and that the tetrahedral sites may similarly be more ordered, although it is not at all clear whether such ordering occurs in the high-temperature, short-duration experimental studies. Thus, as a first approximation, the mixing site distributions are
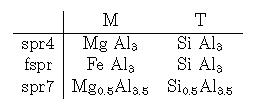
and the ideal activities are then given by
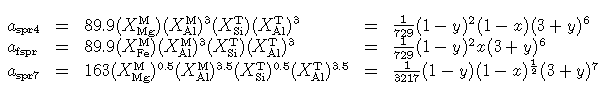
The enthalpies of both these sapphirine end-members depend on this mixing model.
Coded up for THERMOCALC:
% ============= Fe--Mg Sapphirines ========================
spr 3 spr7 1/3217 3 1 1 -1 2 1 1 1 -1 1 3.5 3 1 1 2 7
spr4 1/729 3 1 1 -1 2 2 1 1 -1 1 4 3 1 1 2 6
bulk 1 1 1 1 -2 2
fspr 1/3217 3 1 1 -1 2 1 0 1 1 1 3.5 3 1 1 2 7
bulk 1 2 1 1 -1 2 0 1 2 1
x(spr) 0.15
y(spr) 0.4
|
|