P-T path from Cpx-Hbl-Pl symplectites
|
Eclogites are rocks formed at great depth, as for example when crustal material is subducted during continental collision. Remarkably, later in the collision process, some of this material is returned to the surface from depths of 60 - 120 km or more by mechanisms that are still poorly understood. However, different mechanisms would lead to different pressure-temperature paths during exhumation, so a record preserved in the rocks is very valuable for discriminating among processes. Eclogites of basaltic composition consist mainly of garnet and omphacite, a sodium-rich pyroxene. Na-rich pyroxene is stable only at high pressure, and tends to break down on exhumation to a less Na-rich pyroxene plus plagioclase. Commonly, this takes the form of an intimate intergrowth called symplectite.
This page describes work in progress to extract information about the pressure-temperature path from these intergrowths, using samples from the Western Gneiss complex of Norway. A first report was presented at the Mineralogical Society Winter Meeting in Derby, January 2002. |
Eclogite from western Norway, composed of red garnet and green omphacite. |
Symplectite colonies
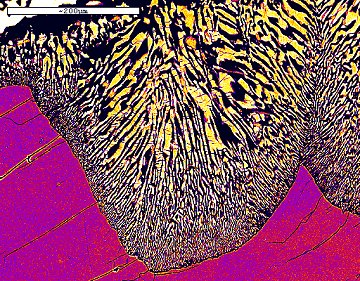
Lobate symplectite colony seen in false-colour
backscattered electron image.
Na-poor pyroxene is yellow, plagioclase black, omphacite
purple.
A symplectite colony nucleates on a grain boundary between two omphacite grains and grows into the grain on one side. The pyroxene in the colony retains structural continuity with the omphacite grain on the other side of the boundary.

We can infer that the symplectite
- Starts growing when the rock is sufficiently out of equilibrium to nucleate the products.
- Stops growing when the temperature is too low for the diffusion of reactant species
Composition trends across the colonies
At this stage of the project, two samples have been studied in detail. One, sample H64a, is from Drage, within the zone of ultra-high pressure (UHP) eclogites on the Stadlandet peninsula, and the other, sample M158, is from the road sections at Krokenakken on Nordfjord. The symplectites were analysed on the JEOL JSM-840A scanning electron microscope equipped with an Oxford Instruments Isis 300 EDS analytical system, using natural and synthetic standards for the major elements and a ZAF correction procedure.
In all samples examined to date, the intergrowth also contains amphibole.

Detail of symplectite colony in backscattered
electron image.
Both pyroxene and amphibole show systematic composition variations across the colony that are consistent with gradually changing conditions during growth. Pyroxene becomes poorer in sodium. Amphibole becomes poorer in Al, Na and Fe3+.
The fact that these trends are preserved suggests that the mineral compositions in the intergrowths have not been changed significantly after growth, and so could be used to retrieve the record of changing conditions during growth.
Pressure-temperature history
To reconstruct the P-T path, we need reliable thermometer and barometer equilibria involving only the phases in the symplectite, i.e. pyroxene, amphibole and plagioclase. There is no free silica in the intergrowths (although it is present in the matrix of the rock), and the activity of H2O is also unknown.
Thermometry
A useful geothermometer is the distribution of Na and Ca between plagioclase and certain sites in amphibole (the "hornblende - plagioclase thermometer") as calibrated by Holland and Blundy (1994). These authors calibrated two thermometers, based on the equilibria
- Ed + 4Qtz = Tr + Ab (the edenite-tremolite thermometer)
- Ed + Ab = Ri + An (the edenite-richterite thermometer)
The second of these thermometers is applicable to the symplectites.

The finer the scale of the intergrowth, the lower the
temperature
recorded by the equilibrium between amphibole and
plagioclase
In practice, temperatures calculated from the silica-saturated edenite-tremolite thermometer are generally slightly lower than, though within error of, the edenite-richterite temperatures. This would not be the case if the activity of SiO2 were significantly less than 1.
Barometry
A convenient and reliable geobarometer would be the equilibrium jadeite + quartz = albite, but for the absence of free silica in the intergrowths. However, it is possible to combine this equilibrium with relationships among the phase components of amphibole in order to eliminate silica, and yet keep the barometer free from dependence on water activity. The new Cpx-Pl-Hbl barometer equilibrium is
4 jadeite + tremolite = 3 albite + edenite
This equilibrium can be calibrated using the latest version (May 2001) of the thermodynamic data set described by Holland and Powell (1998). Data are available for pargasite and tschermakite, rather than for edenite, so the calibrated equilibrium is of the form 8jd + tr + ts = 2parg + 6 ab. A linearised calibration, centred on 650°C, 12 kbar, is:
0 = 59.34 - 0.35064T(K) + 11.924P(kbar) + RTlnK
The activities of amphibole end members are calculated according to Dale et al. (2000). Tim Holland's program AX provides a convenient way of calculating these. The activity of jadeite uses a two-site non-ideal model, formulated either after Holland (1990) or after Holland and Powell (1996). The albite component in sodic plagioclase can be assumed to be ideal.
P-T arrays for the two samples, made by pairing the Cpx-Pl-Hbl pressures with temperatures from the edenite-richterite thermometer, are shown below. They are consistent with a history of cooling during exhumation. Also shown are pressures calculated from the jadeite-quartz-albite barometer, at the same tempratures. These ought to represent maximum pressures for the assemblage in the presence of free quartz. The fact that the array falls at slightly lower pressure suggests that the Cpx-Pl-Hbl barometer slightly over-estimates the true pressures.
Synthesis
The eclogite H64a formed at about 30 kbar, and M158 probably at about 22 kbar (about 75 km depth). In the figure below the symplectite P-T arrays are compared with the peak conditions and suggested model exhumation paths.
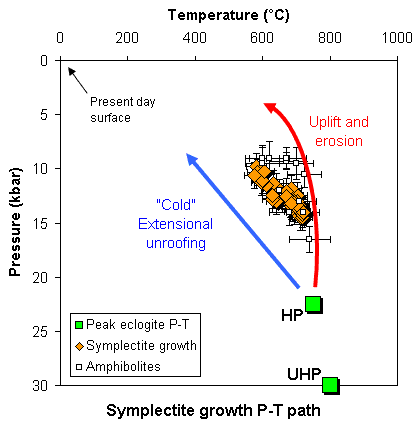
The symplectite P-T array appears to lie at an angle to smooth exhumation trajectories. If not an artefact of the P-T calculation, this could imply a change to slower exhumation rates, or a change in exhumation mechanism, after an initial period of near-isothermal decompression.
In addition, the P-T arrays can be compared with P-T conditions calculated for amphibolite-facies gneisses derived from the refoliated margins of eclogite bodies (data from Alice Wain's 1998 thesis). These P-T points were derived by combining the hornblende-plagioclase thermometers with THERMOCALC average-P calculations based on assemblages containing hornblende, epidote, biotite, plagioclase and quartz +/- clinopyroxene. The four amphibolite samples that deviate the most from the symplectite array are from UHP localities. The remaining samples delineate an array similar to that shown by the symplectites.
References
Dale, J., Holland, T. and Powell, R. (2000). Hornblende-garnet-plagioclase thermobarometry; a natural assemblage calibration of the thermodynamics of hornblende. Contributions to Mineralogy and Petrology, 140 (3), 353-362.
Holland, T.J.B. (1990). Activities in omphacitic solid solutions: an application of Landau theory to mixtures. Contributions to Mineralogy and Petrology, 105, 446-453.
Holland, T. and Blundy, J. (1994). Non-ideal interactions in calcic amphiboles and their bearing on amphibole-plagioclase thermometry. Contributions to Mineralogy and Petrology, 116, 433-447.
Holland, T. and Powell, R. (1996). Thermodynamics of order-disorder in minerals; II, Symmetric formalism applied to solid solutions. American Mineralogist, 81, 1425-1437.
Holland, T. J. B. and Powell, R. (1998). An internally consistent thermodynamic data set for phases of petrological interest. Journal of Metamorphic Geology, 16, 309-343.
Created/updated: 22 February 2003