Equilibrium calculation and real metamorphic process: scenes from a troubled relationship
Metamorphic Studies Group Research in Progress 2010
(keynote);
Interplay between Thermodynamics, Kinetics and Deformation in Metamorphism,
GeoCanada 2010 (invited talk).

My daughter Helen drew this cartoon to illustrate the principle of the title. Equilibrium, on the left, believes metamorphic products should always follow ordered patterns governed by the logo on his t-shirt. He looks disgruntled because Process, on the right, is busy explaining why she won't do this metamorphism thing exactly the way he plans it. She clearly has her own style, and while her successful products may sometimes take unexpected forms, you can bet that she has found efficient ways of making them, because she wouldn't tackle any job that involved too much effort to get it started.
Equilibrium and Process seem like an ill-matched couple, but they need each other, and we have to understand the point of view of each of them in order to grasp the principles of metamorphism.
What follows is an extended abstract of the GeoCanada lecture (with some updated figures).
Summary
Ongoing developments in thermochemical data sets and calculation software have led to the routine use of calculated equilibrium phase diagrams, and in particular P-T pseudosections, as a tool for investigating the P-T evolution of metamorphic rocks. However, the real processes that transform metamorphic rocks take place away from equilibrium, and are governed by the kinetic controls on nucleation, interface reaction, and mass transfer.
This contribution explores how the microstructural and micro-compositional record in rocks may, or may not, be reconcilable with calculated phase diagrams in the relevant systems. Examples are drawn from metapelites in the High Himalaya, from the aureole of the Bushveld Complex, and from Alpine metabasic eclogites. In practice we find that although the predicted equilibrium assemblage is commonly approached, the reaction pathways can be strongly affected by nucleation barriers, compositional fractionation effects, and the slow decomposition of reactant phases.
Introduction
The advent of self-consistent data sets for systems closely relevant to natural rocks and the steady improvement of software tools for calculating phase diagrams (e.g. Powell et al., 1998) are transforming our ability to visualize the equilibrium state of metamorphic systems. P-T pseudosections, for example, are routinely used to constrain P-T conditions and infer P-T-time paths.
The real processes that transform metamorphic rocks, however, take place away from equilibrium, and are governed by kinetic constraints. Non-equilibrium effects are potentially at play in all three fundamental processes of prograde metamorphic change: barriers to nucleation of new phases, sluggish rates of interface reaction, and limits to the effectiveness of mass transfer. Moreover, the pseudosection approach requires that the bulk composition is defined, and yet the effective composition of the reacting system is governed by these same kinetic factors. In particular, refractory porphyroblasts fractionate material from the reacting system, and may also be slow to release material when they become reactants. Illustrations of these effects are taken from a range of case studies in which the microstructural and micro-chemical record is compared with calculated phase relationships.
Case study 1: Everest metapelite
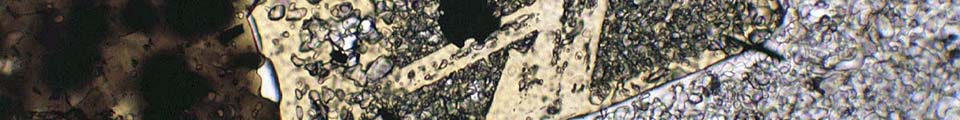
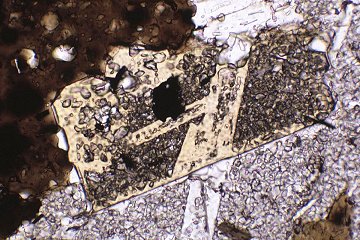
Metapelitic rocks in the footwall of the South Tibetan Detachment System at Mt Everest contain a detailed record of their metamorphic evolution. Microstructural analysis of a lower sillimanite zone schist reveals that garnet growth entirely predates the main S2 matrix fabric, which formed under staurolite-zone conditions. The record of compositional zoning in garnet also reveals that it grew only under garnet zone conditions on the trajectory shown by the hollow arrow on Figure 1, the remainder of the path being deduced from other data. In contrast to the equilibrium prediction, garnet failed to contribute to staurolite growth, and ultimately experienced corrosion under sillimanite-zone conditions without re-equilibration of the garnet rims, despite the high peak temperature of around 640°C (Figure 1). It follows that any attempt to calculate "peak" P and T by conventional thermobarometry using the full assemblage Grt-St-Sil-Bt-Ms-Pl-Qtz is doomed to failure.
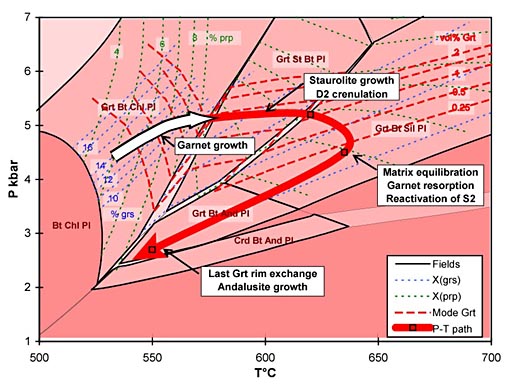
Figure 1: P-T pseudosection in the system NCKFMASH for a sillimanite-zone Everest Series metapelite. The hollow arrow shows the growth interval of garnet calculated from compositional zoning profiles.
Case study 2: Aureole of the Bushveld Complex
Shales of the Timeball Hill Formation (Pretoria Group) experienced a static but long drawn-out metamorphism with a peak at 550°C, 3 - 3.5 kbar in the thermal aureole beneath the 8 km thick mafic Rustenburg Layered Series. Mineral growth microstructures, preserved in exquisite detail, reveal that porphyroblastic staurolite, cordierite, biotite and andalusite appeared in an overlapping sequence entirely at odds with the equilibrium phase diagram (Figure 2), apparently as a consequence of delayed nucleation of andalusite and the parallel operation of several metastable reactions (Waters and Lovegrove, 2002). The full range of microstructures can be attributed to nucleation barriers differing among the product phases, evolution of the effective bulk composition (EBC) by fractionation of the newly-grown porphyroblasts, and slow dissolution of reactant chloritoid compared to matrix sheet silicates. Nevertheless, the final assemblage at the metamorphic maximum approaches a possible equilibrium one.
(a)
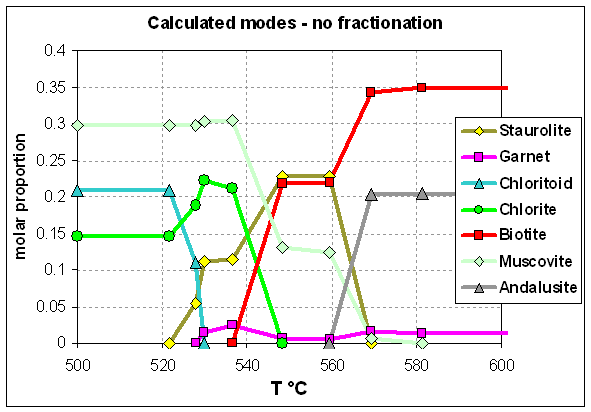
(b)
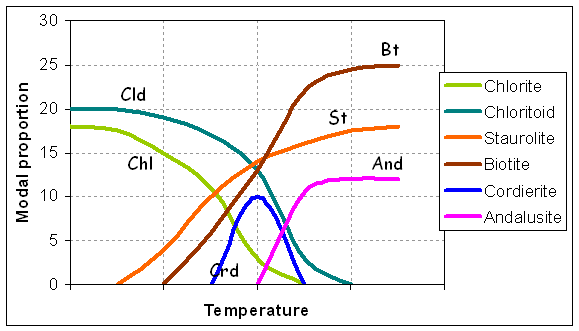
Figure 2: (a) Calculated, and (b) observed sequence of growth and relative modal proportions of minerals in Timeball Hill metapelite, after Waters and Lovegrove (2002).
Case study 3: Alpine metabasic eclogites
Eclogites from the Monviso unit, Italian Western Alps, shed further light on fractionation processes and the evolution of a rock's effective bulk composition. The dominant minerals are omphacitic clinopyroxene and garnet, both of which are refractory and show compositional zoning related to growth along a P-T trajectory. However, because the rocks were undergoing dynamic recrystallisation, fractionation of mineral core compositions out of the EBC is not fully effective. In garnet, networks of vein-like Mg-rich domains, probably representing fluid pathways along healed fractures, penetrate and transform early garnet cores, commonly leading to atoll structures. In omphacite aggregates, migrating grain boundaries sweep across the interiors of old grains, recycling core material back into the EBC. Estimating the EBC at different stages of the P-T evolution is a challenging task!
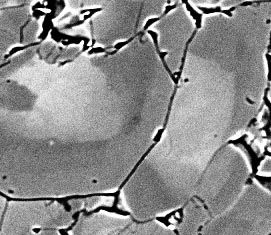
Figure 3: Backscattered electron micrograph showing growth-zoned omphacite in an aggregate that has undergone dynamic recrystallisation. Grain at left advanced into the grain at right, its medium-grey rim truncating the bright (Fe-rich) core and dark (Na-rich) outer zone of the right-hand grain. Width of view approx 120 μm.

Figure 4: Backscattered electron micrograph showing growth-zoned garnet modified by darker vein-like Mg-rich domains. Width of view approx 0.5 mm.
Conclusions
In practice, therefore, we find that although calculated phase diagrams provide a valuable frame of reference for interpreting the P-T evolution of metamorphic rocks, the actual reaction pathways can be strongly affected by disequilibrium processes. Moreover, the effective bulk composition of a rock undergoing metamorphism is not always easily established, and may change significantly during the P-T evolution. A detailed study of microstructural and micro-compositional patterns may be needed to interpret the mineral assemblage evolution, but a combination of this process-oriented approach with equilibrium calculation can yield unexpected insights into metamorphic processes.
Acknowledgements
I am grateful to the organizers of this special session for their invitation to contribute, and especially to Dave Pattison for long discussions on kinetic matters. Studies in the Everest Himalaya involve collaboration with Mike Searle, Rick Law, John Cottle and Micah Jessup; Dan Lovegrove contributed to the Bushveld aureole work; and Ryan Langdon and Samuel Angiboust are involved with the ongoing study of the Monviso eclogites.
References
Powell, R., Holland, T.J.B., and Worley B., 1998. Calculating phase diagrams involving solid solutions via non-linear equations, with examples using THERMOCALC: J. Metamorphic Geol., 16, 577-588.
Waters, D.J., and Lovegrove, D.P., 2002. Assessing the extent of disequilibrium and overstepping of prograde metamorphic reactions in metapelites from the Bushveld Complex aureole, South Africa: J. Metamorphic Geol., 20, 135-149.