Practical Aspects of Mineral Thermobarometry
Thermobarometer Calibration
The aim of this section will be to cover briefly such topics such as
- Conditions of heterogeneous equilibrium
- The equilibrium constant and its P-T dependence.
- A simple geothermometer: garnet-biotite Fe-Mg exchange
- A geobarometer based on a net-transfer reaction with large volume change
- How thermometers and barometers are calibrated
- Self-consistent thermodynamic data sets and their application to thermobarometry
You can read about these subjects in Spear 1993, chapter 8, pp. 241-244, and chapter 15, pp. 511-537.
Here's some provisional content, from lecture course handouts:
Condition for heterogeneous equilibrium
[Spear, p. 511]. A balanced chemical reaction such as Grs + 2 Ky + Qtz = 3 An could also be written in the form 3 An - Grs - 2 Ky - Qtz = 0, reminding us that the mass of each system component is conserved, and that reaction coefficients can be positive ("products") or negative ("reactants"). At equilibrium, a similar relationship holds among the chemical potentials of phase components (n are the reaction coefficients and there are m phase components):
![]()
i.e. there is no free energy difference between "reactants" and "products".
Expand the expression for heterogeneous equilibrium
[Spear, p. 512]. Adding up, for all the phase components, the expressions µi = µ°i + RTln(ai), gives us
![]() GR = 0 =
GR = 0 =
![]() G0 + RTlnK
G0 + RTlnK
where K is the equilibrium constant or activity
product, which depends on measured phase compositions.
![]() G0
is the standard state free energy change - something we can calculate
if we have thermochemical data. Expanding
G0
is the standard state free energy change - something we can calculate
if we have thermochemical data. Expanding
![]() G0 to see what thermochemical data are
needed, we get:
G0 to see what thermochemical data are
needed, we get:
![]()
The subscripts and limits reflect the fact that thermochemical data are generally tabulated at 298K and 1 bar.
Linear approximation for solid-solid reactions
[Spear, p. 523]. ![]() CP = 0.
The
CP = 0.
The ![]() CP terms adjust
CP terms adjust
![]() H and
H and
![]() S for different P and T. The heat
capacities of solid phases vary sympathetically, so
S for different P and T. The heat
capacities of solid phases vary sympathetically, so
![]() CP is small, and can often be ignored,
particularly over small ranges of temperature.
CP is small, and can often be ignored,
particularly over small ranges of temperature.
![]() V constant. The volume integral brings the
enthalpies from their reference pressure P0 (1 bar)
up to the pressure of interest (usually several thousand bars).
V constant. The volume integral brings the
enthalpies from their reference pressure P0 (1 bar)
up to the pressure of interest (usually several thousand bars).
![]() V is not a function of P or T if
all solids are roughly equally compressible, so the volume integral
becomes
V is not a function of P or T if
all solids are roughly equally compressible, so the volume integral
becomes ![]() V(P - P0). Also,
P0 is negligible compared to P.
V(P - P0). Also,
P0 is negligible compared to P.
This gives us the greatly simplified expression
![]()
which, for a given value of lnK, is the equation of a straight line on a P-T diagram. The curve for lnK = 0 (i.e. K = 1) is the curve for pure phases, such as might be determined in the experimental laboratory. Other values of lnK displace the curve across the P-T diagram.
Dependence of lnK on T and P
[Spear, pp. 515-6]
![]()
![]()
where ![]() S and
S and
![]() V are calculated at the
P and T of interest. Also remember the
Clausius-Clapeyron equation:
V are calculated at the
P and T of interest. Also remember the
Clausius-Clapeyron equation:
![]() ; or, more strictly,
; or, more strictly,
![]()
Therefore, a good geobarometer needs a large
![]() V, in
order to have a large sensitivity to P and for curves of
constant K to have a low slope on the P-T
diagram.
V, in
order to have a large sensitivity to P and for curves of
constant K to have a low slope on the P-T
diagram.
Geothermometry and geobarometry using multivariant equilibria
At equilibrium
DGP,T = DH1,T - T.DST + (P-1).DV + RT.lnK = 0
K is the equilibrium constant, the product of the activities of end members in the phases of variable composition.
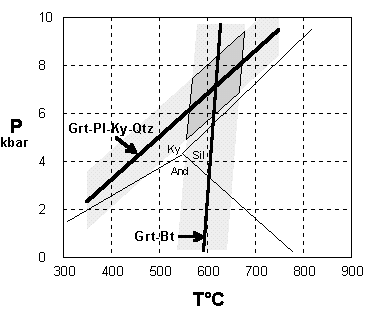
The Grt-Pl-Ky-Qtz geobarometer
Ca3Al2Si3O12 + 2Al2SiO5 + SiO2 = 3CaAl2Si2O8
grossular(Grt) + kyanite + quartz = anorthite(Pl)
Substituting thermochemical data, dividing through by the gas constant R, and expanding K gives the calibration:
7635 - 19.66T + 0.7963(P-1) + 3Tln(XCa,Pl /XCa,Grt) = 0
DV is large, because of the density difference between open feldspar and close-packed garnet and kyanite structures. The P-T slope is relatively low, making this equilibrium useful as a barometer. Uncertainty on calculated P is about ± 1.3 kbar.
The Grt-Bt Fe-Mg exchange thermometer
Fe3Al2Si3O12 + KMg3AlSi3O10(OH)2 = Mg3Al2Si3O12 + KFe3AlSi3O10(OH)2
Almandine (Grt) + Phlogopite (Bt) = Pyrope(Grt) + Annite (Bt)
DG for cation exchange is small, and the calibration here is fitted to exchange equilibrium experiments
6266 - 2.35T + 0.029(P-1) + 3TlnKD = 0
KD is most simply expressed as (Mg/Fe)Grt /(Mg/Fe)Bt
DV is very small, and cation exchange equilibria are generally insensitive to pressure. The usually-quoted uncertainty on calculated T is ± 50°.
Application
An amphibolite-facies metapelitic schist from the Eastern Alps contains garnet of composition (% end members) Alm80Sps3Prp13.5Grs3.5 , biotite with Mg/Fe = 0.91, and plagioclase An15 , together in apparent equilibrium with kyanite and quartz. From these data we calculate the two P-T curves. Their intersection (with a surrounding box of uncertainty) gives us an estimate of the conditions of equilibration.
Self-consistent data-sets
[ Material to incorporate from Petrology course lecture slides ]
This page last modified 12 October 2004
