Microstructures of Metamorphic Rocks
Chemical reactions in rocks
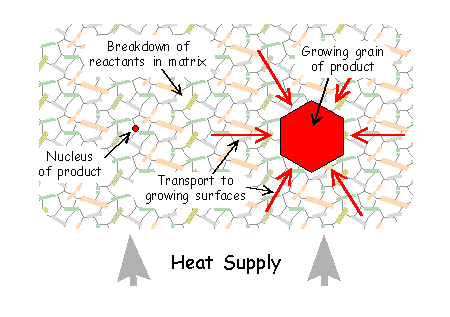
A metamorphic rock consists of individual grains of several solid minerals (through which transport of material is relatively difficult) and a network of grain boundaries, which at the time of metamorphism may have held an aqueous fluid, providing pathways for transport through the rock.
For a new mineral to appear by a chemical reaction, a number of processes have to operate in concert:
- Nucleation: nuclei (embryo crystals) of the new mineral appear.
- Interface reactions - dissolution: reactant minerals break down, their chemical constituents going into solution
- Interface reactions - growth: material is added onto the nuclei to build larger crystals
- Mass transfer: material is transported through the rock from sites of breakdown to sites of growth
Each of these steps involves surmounting one or more energy barriers.
Overstepping
During prograde metamorphism, heat is added to the rocks, increasing their temperature. Eventually the equilibrium temperature of the reaction of interest is exceeded, and there is a chemical driving force favouring growth of the products. The driving force is proportional to the amount the equilibrium temperature has been overstepped or exceeded. This concept of overstepping is a useful one.
The greater the overstepping, the higher the proportion of successful attempts to cross the energy barriers in the reaction steps. The rates of the individual steps, therefore, depend on the overstepping, but they are also controlled by other factors which depend on the local environment or on the history of the rock. For example, transport rates will be more rapid in the presence of intergranular fluid than in its absence, and the overall rate of growth (which depends on the surface area of growing grains) will be greater if the new material consists of many small grains than if it forms few large ones.
This project: measuring the barrier to nucleation
The processes also interact with each other in complex ways which affect the microstructure of the rock. The simplest of these interactions accounts for the presence of porphyroblasts in slowly-heated regional metamorphic rocks, in contrast to the fine crystals of a contact-metamorphic hornfels (see next panel).
We could extract information about the rates of metamorphic processes from rock textures, if we knew the rate laws for the individual processes and could model how they interact. In practice the stumbling block is our inadequate knowledge of the nucleation step. Clearly nothing can happen until product crystals have nucleated, and the overstepping required to do this controls how far metamorphic systems are from chemical equilibrium. This project aims to measure the overstepping and quantify the nucleation step indirectly, by analysing the microstructures of metamorphic rocks of known thermal and chemical history.