Practical Aspects of Mineral Thermobarometry
Garnet
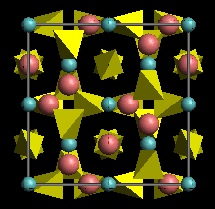
Sites
Garnets have the basic formula
X3Y2Si3O12
where
- X is an 8-co-ordinated site containing divalent cations (Fe2+, Mg, Mn Ca).
- Y is an octahedral site containing trivalent cations (Al, Fe3+, Cr)
End Members
The common end members are
| Pyrope | Mg3Al2Si3O12 |
| Almandine | Fe3Al2Si3O12 |
| Spessartine | Mn3Al2Si3O12 |
| Grossular | Ca3Al2Si3O12 |
| Andradite | Ca3Fe3+2Si3O12 |
Stoichiometry
In crustal metamorphic rocks, Si should be 3 cations, within error. If it isn't, check the microprobe calibration.
In the garnets typical of metapelitic and metabasic rocks, Al is the dominant trivalent cation. It should not exceed 2 cations per formula unit. Most commonly it falls between 1.95 and 2.00, and the deficit is presumed to be made up by ferric iron.
The divalent cations should total 3, though there may be a small excess if ferric iron is present. The ideal cation total is 8. A small excess may indicate ferric iron.
Fe recalculation, and problems regarding Fe-rich minerals
To recalculate ferric iron, normalise to 12 oxygens and 8 cations, using the method of Droop (1987). The ferric iron is usually assigned to the andradite end member, and it is unusual for Fe3+ to exceed Ca.
You may already have noticed that garnet analyses performed on many microprobe systems have rather high analytical totals, of 101% or more. This appears to be a problem common to Fe-rich minerals (it is sometimes seen in Fe-rich pyroxenes too), and it suggests that the correction procedures for such minerals may not be entirely adequate. The problem is not merely an overestimation of Fe, as the stoichiometry is normally acceptable. Be suspicious about the analysis quality, however, if you find that after ferric iron recalculation, the total divalent cations are significantly less than 3.
Compositional zoning in garnets
Garnets commonly show compositional zoning, which results from slow diffusion in the garnet lattice. Diffusion of the divalent cations in garnet is negligible at low and medium grade, so that the interior of a garnet is isolated from the rock matrix, but may become extensive enough for garnet to reach a homogeneous equilibrium composition at high grade. Two fundamentally differing styles of zoning pattern can be distinguished.
- Growth zoning, in which a growing garnet does not homogenise the composition of its interior, but adds successive shells of material whose composition reflects equilibria and processes in the rock matrix. Such garnets tend to have Mn and/or Ca-rich cores, with XMg increasing steadily towards the rim. This is a fractionation process. This style is found in garnets which have grown at temperatures below about 650°C.
- Diffusional zoning, in which a pre-existing garnet is modified in composition by exchange of material with the rock matrix, and the compositional gradients are frozen in on cooling before a new homogeneous composition can be achieved. The usual result is a garnet with a depletion in Mg, and commonly also an enrichment in Mn, at the rim of the garnet. This style is found in garnets which have experienced temperatures above about 600°C.
![[image]](L429grt.gif)
The garnet above, from a lower-grade part of the High Himalayan slab, shows prograde growth zoning.
![[image]](zd52grt.gif)
This garnet, from the high-grade part (Sil zone) of the High Himalayan Slab, shows retrograde diffusional zoning.
For further examples of zoned garnets and the complex features they may reveal, see the collection of microprobe element maps made at the University of Massachusetts microprobe laboratory.
This page last modified 12 October 2004
